Alternative splicing
Expression of genetic information requires removal of interrupted sequences (introns) and reunion of gene fragments (exons), which happens at the RNA level via splicing (Nobel prize in Physiology and Medicine 1993 shared by Richard Roberts and Phillip Sharp). Splicing is a necessary processing step for various types of RNA, including pre-messenger RNA (pre-mRNA), tRNA, rRNA and long non-coding RNA (lncRNA).
How pre-mRNA splicing occurs has been extensively studied with biochemical and genetic approaches. The current biochemical model of the splicing reaction is centered around the step-wise assembly of spliceosome on pre-mRNA, which involves ordered recruitment of five small nuclear RNAs (U1, U2, U4, U5 and U6 snRNA), or more precisely small nuclear ribonucleic protein (snRNP) complexes. The spliceosome is a very dynamic structure in which both RNA-RNA base-paring and RNA-protein interactions are formed and broken constantly. The dynamic nature of the spliceosome is believed to expose multiple quality checkpoints as well as regulatory entries.
Changes in splice site choices (i.e. exon-intron boundary) result in different spliced variants from a single gene. This phenomenon, alternative splicing, is an important mechanism for regulation of gene expression. It significantly increases the transcriptome diversity and the coding capacity of our genomes with a finite number of genes. Alternative splicing is particularly common in higher eukaryotes. In human, about 95% of multiexonic genes produce alternatively spliced isoforms. Many principle modes of alternative splicing have been described (Zheng, WIREs RNA 2020).
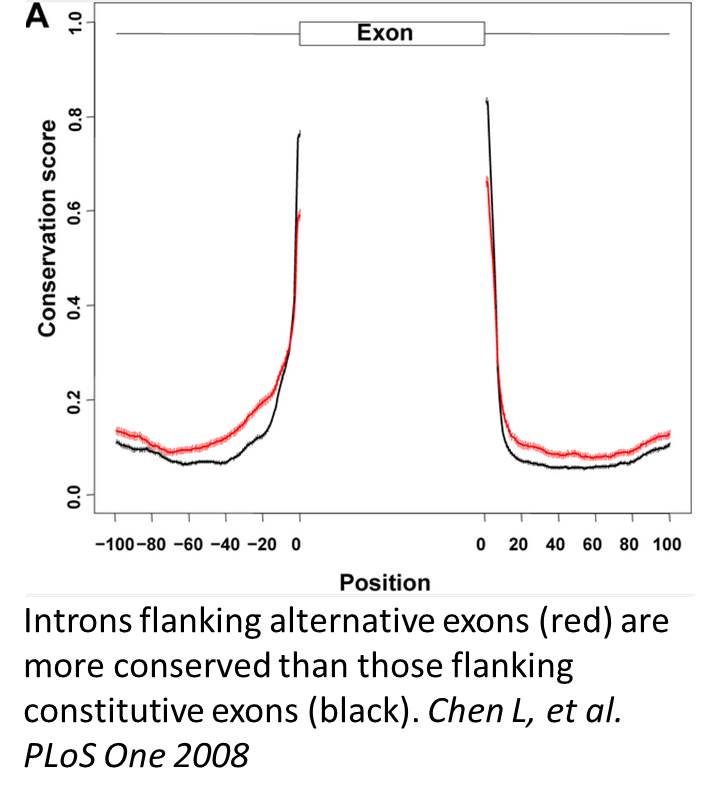
Regulated splicing is most common through the action of trans-acting RNA binding proteins (RBPs) that recognize cis-acting sequence elements in the pre-mRNA. Assembly of RBPs on pre-mRNA forms regulatory ribonucleoprotein complex (RNP) and subsequently affects splice site selections and/or spliceosome assembly. Many of the cis-acting elements are in flanking introns. Introns flanking alternative exons are generally more conserved than those flanking constitutive exons (Chen et al., PLoS One 2008). This implies functional importance of some of these regulatory elements across species. Indeed, we used position-specific conservation pattern to predict de novo verifiable alternative exons (Chen et al., PLoS One 2008).
The alternative splicing landscape of mammalian neuronal differentiation
Disruption of the splicing regulatory processes has been found in schizophrenia, autism spectrum disorders, Amyotrophic lateral sclerosis (ALS), frontotemporal dementia, and multiple neuromuscular dystrophy. mammalian neurons use a repertoire of alternative splice sites different from most cell types. The selections of these alternative splice sites change transcript structure and coding and are often determined during neuronal differentiation by RNA binding proteins. However, the roles of most splicing regulators in the brain are largely unclear. We also know little how and why a neuron choose particular exons. How do these alternative exons contribute to the neuronal phenotypes and the establishment of neural circuits? How is neuron specific splicing dynamically regulated in health diseases? How does neurons generate diversity from a relatively small number of primary transcripts?
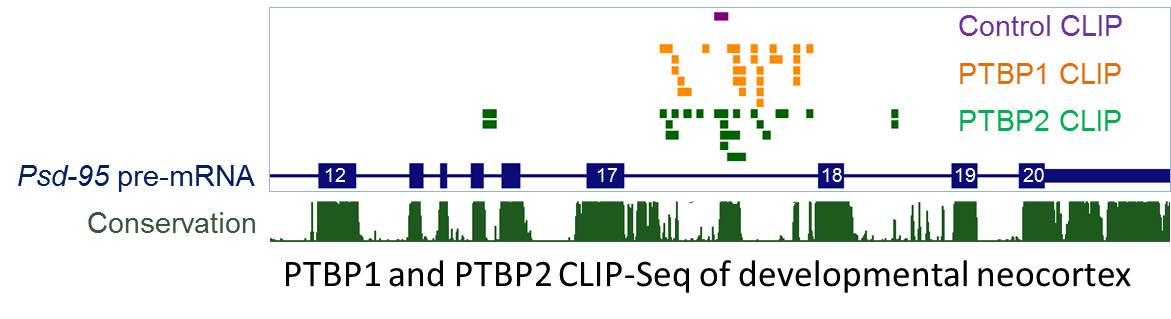
We focus on one family of splicing regulators--the polypyrimidine tract binding proteins (PTBP) that preferentially bind to CU rich elements in pre-mRNA. We and others have found that as neural progenitors differentiate, PTBP1 is down-regulated and its paralog PTBP2 is induced. However, as neurons mature, PTBP2 expression decreases (Zheng et al., Nature Neuroscience 2012). The down-regulation of PTBPs is necessary for synapse maturation, in part because PTBPs control a neuronal exon of Psd-95 to inhibit overall expression of PSD-95, a key scaffold protein of excitatory post-synaptic density that promotes synapse maturation. Using CLIP-Seq, we found that both PTBP1 and PTBP2 bind to conserved intronic sequences upstream of Psd-95 exon 18 in embryonic brains.
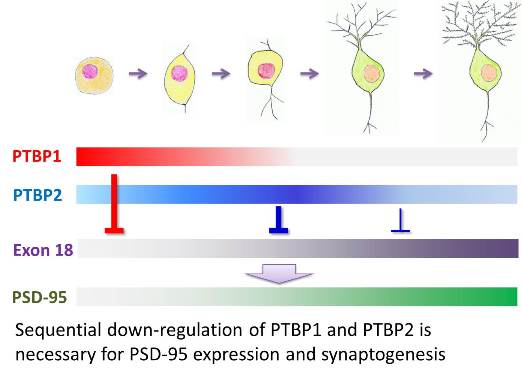
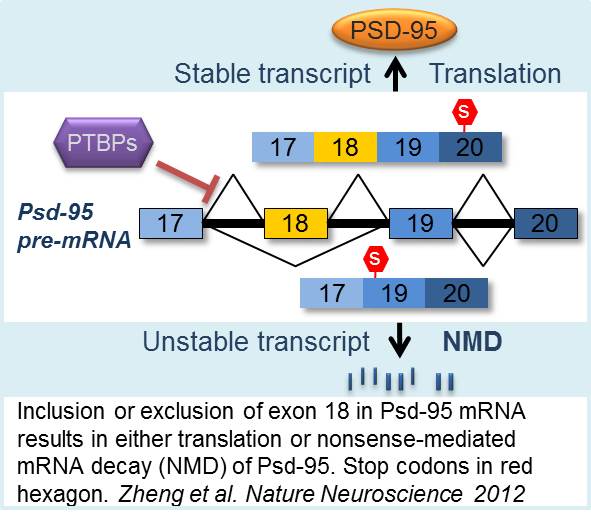
In immature neurons, the inclusion of exon 18 in Psd-95 mRNA is inhibited first by PTBP1 then by PTBP2. Skipping exon 18 leads to nonsense-mediated mRNA decay (NMD) of the Psd-95 transcripts without productive translation. Mature neurons express exon 18 and PSD-95 proteins thanks to reduced expression of PTBPs. This shows an example of how a neuron specific exon is regulated during development to control expression of an essential synaptic protein and synaptogenesis. It also demonstrates a fundamental principle that genetic message is not definitely established when RNA is first transcribed; instead, it is the splicing pattern that determines the nature of the final mRNA product.
Alternative splicing acts as an on/off switch of gene expression
The coupling of alternative splicing to NMD (AS-NMD) allows a splicing choice to act as an on/off switch for a final gene product, as seen for Psd-95. This is rather different from the commonly perceived outcomes of alternative splicing, i.e. modifying protein structures and activity. Interestingly, Psd-95 is transcribed but its protein is absent in non-neuronal cells where PTBP1 is expressed at a high level. Thus, splicing regulation of exon 18 enforces neuronal specific expression of PSD-95 proteins.
